Halneuron®
for Pain Management
About Halneuron®
Halneuron® is WEX’s primary product under development. Halneuron’s active pharmaceutical ingredient is Tetrodotoxin (TTX), a small molecule known to block voltage-gated sodium channels on neurons and prevents pain by inhibiting the initiation and conduction of nerve impulses in the peripheral nervous system which is associated with pain.
Halneuron is administered by subcutaneous injection and has been tested on over 700 people to date. Halneuron (TTX) has been tested on people with moderate-to-severe cancer pain as well as people with moderate-to-severe chemotherapy-induced neuropathic pain where clinical data to date has shown varying degrees and duration of pain relief, and a reduction in opioid consumption.
Halneuron (TTX) is in late-stage clinical development for the treatment of chemotherapy induced neuropathic pain and cancer pain. Halneuron (TTX) is also being developed for the treatment of other pain indications.
Halneuron® as
an alternative to
narcotics and opioid
pain medication
Halneuron® as an alternative to narcotics and opioid pain medication
The current gold standard for moderate to severe pain treatment are opioids but opioids have limitations and serious risks associated with continued use including respiratory, gastrointestinal and central nervous system side effects. Overtime, opioids can become ineffective and expose patients to risks of addiction and tolerance.
Clinical data has shown Halneuron provides pain relief in certain moderate to severe pain conditions without the opioid side effects. Halneuron has an acceptable safety profile with no evidence of addiction or tolerance. Some patients have demonstrated a long duration of pain relief as well as a meaningful reduction in their opioid consumption.
What is Halneuron®?
Halneuron® is Tetrodotoxin (TTX), a sodium channel blocker
TTX works as a painkiller by blocking NAv 1.7, a sodium channel responsible for pain
TTX binding and blocking NAv 1.7 are well understood
How does Tetrodotoxin work?
Pain signals are nerve impulses that travel along a nerve as electrical signals generated by the movement of sodium ions through ion channels on the surface of nerve cells.
TTX binds to and blocks sodium ion channels on the nerve cell surface, reducing the movement of sodium ions, thereby reducing the conduction of pain signals.
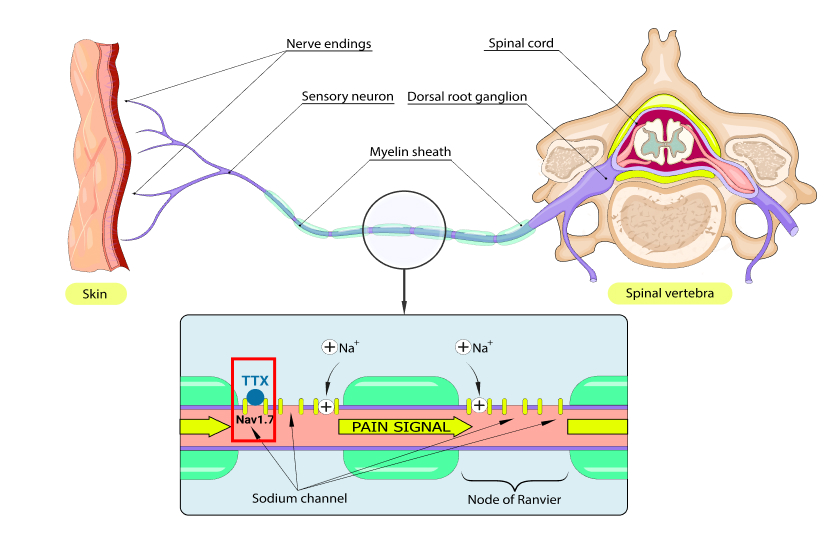
NAv 1.7 is Directly Responsible For Feeling Pain
The sodium channel NAv 1.7 is responsible for the perception of pain. This is well supported in peer review research over the past decades1,2,3
Two health conditions regarding abnormal expression of NAv 1.7 and their effects on the perception of pain is well-documented
Feel No Pain
Congenital Insensitivity to
Pain Syndrome
- Rare genetic mutation that causes an absence of a NAv 1.7 sodium channel
- Condition that prevents the ability to perceive pain
Hypersensitivity to Pain
Erythromelalgia:
Severe Pain Syndrome
- Hypersensitivity to pain
- Mutations in the sodium channel NAv 1.7 produce pain syndromes
- Characterized by feeling intense, burning pain primarily affecting one’s feet and hands
Notes:
- Dib-Hajj SD, Yang Y, Black JA, Waxman SG. The Na(V)1.7 sodium channel: from molecule to man. Nat Rev Neurosci. 2013 Jan;14(1):49-62.
- Han C, Rush AM, Dib-Hajj SD, Li S, Xu Z, Wang Y, Tyrrell L, Wang X, Yang Y, Waxman SG. Sporadic onset of erythermalgia: a gain-of-function mutation in Nav1.7. Ann Neurol. 2006 Mar;59(3):553-8.
- Faber CG, Hoeijmakers JG, Ahn HS, Cheng X, Han C, Choi JS, Estacion M, Lauria G, Vanhoutte EK, Gerrits MM, Dib-Hajj S, Drenth JP, Waxman SG, Merkies IS. Gain of function Naν1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. 2012 Jan;71(1):26-39.
Pain Clinical Trial Results
Latest Clinical Trials Investigating Halneuron®
- Studies using Halneuron® for managing pain have shown encouraging clinical results
- Lead indications for Halneuron® include two recent studies:
- Phase III Cancer Related Pain (“CRP”) Clinical Trial
- Phase II Chemotherapy-Induced Neuropathic Pain (“CINP”)
Phase III Cancer Related Pain
Clinical Trial
Phase III Cancer Related Pain Clinical Trial
- Tested for efficacy and safety of TTX for moderate to severe inadequately controlled CRP
- Randomized, double-blind, placebo-controlled, parallel-design, multicenter, trial
- Patients enrolled: 165
- Statistically significant pain reduction
Some patients demonstrated pain relief for more than 30 days post injection period
TTX showed an acceptable safety profile in cancer patients.
Phase II Chemotherapy-Induced
Neuropathic Pain
- Primarily a dose finding trial but also evaluating potential efficacy and safety of TTX in patients with CINP
- Randomized, double-blind, dose-finding, placebo-controlled, multicenter study
- Patients enrolled: 125
Optimal therapeutic dose to treat CINP established
TTX showed an acceptable safety profile in CINP patients.
Phase III Cancer Related Pain Clinical Trial
Phase III CRP: Clinical Trial Design
Phase III CRP - Patient Population
| TTX1 | Placebo2 | Total | |
|---|---|---|---|
| # of Patients Enrolled | 77 | 88 | 165 |
|
Patients Completed Study Patients Discontinued Study |
64 13 |
83 5 |
147 18 |
|
Patients With Moderate Pain Patients With Severe Pain |
9 63 |
12 76 |
21 144 |
| Average Pain Score Before Treatment | 7.6 | 7.6 |
- All patients were on best standard of care3 with half receiving Placebo and the other half receiving TTX
- TTX treatment involved 2 injections a day for 4 days (total of 8 doses)
- All patients recorded their pain score in two distinct periods with extended follow up:
- Pain response first measured from days 5 to 8 post injection (Early Post-Injection Period)
- Pain response also measured from days 9 to 15 post injection (Late Post-Injection Period)
- Follow up conducted after late post-injection period to measure any prolonged effect from TTX on pain
Phase III CRP: Pain outcome p-value < 0.05
- There was a statistically significant improvement in pain outcome for TTX
- 51% of patients receiving TTX experienced a reduction in pain; vs
- Only 35% of patients in the placebo group recorded a reduction in pain
Pain Outcome – Co-Primary Endpoint
(Pain Intensity Difference and/or Opioid Use)
| TTX1 | Placebo2 | Difference | |
|---|---|---|---|
| Responder |
3351%
|
2935% |
16% |
| Non-Responder |
3249%
|
5565%
|
|
| Total | 65 | 84 | |
| 95% C.I. | 0.4- 32.1 | ||
| p-value | 0.046 |
A “Responder” is defined as a patient who has:
- a mean reduction in pain
- intensity of ≥ 30%; or
a decrease of at least 50% of opioid use
- TTX + Standard of Care.
- Placebo + Standard of Care.
- Standard of Care is defined as optimized opioid and co-analgesic therapy specific to each patient.
Phase III CRP: TTX reduced worst pain
- There were more patients that demonstrated at least 30% pain reduction in the TTX treatment group over the Placebo group in both Early Post Injection Period (“EPIP”) and Late Post Injection Period (“LPIP”)
- Furthermore, using any percentage of pain relief, TTX always demonstrated superior pain reduction over Placebo.
Early Post Injection Period (Days 5-8)
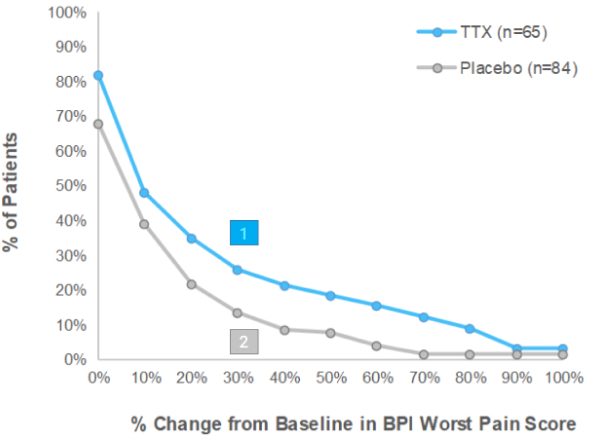
% of patients in group showing ≥30% reduction in BPI2
Worst Pain Score in EPIP
124.6% of patients on TTX, vs
211.9% of patients on Placebo
Late Post Injection Period (Days 9-15)
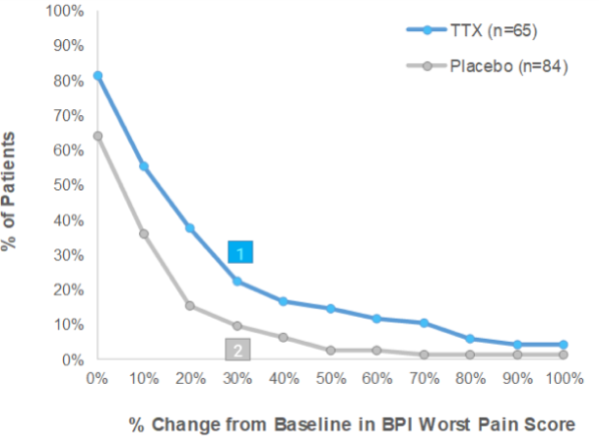
% of patients in group showing ≥30% reduction in BPI2
Worst Pain Score in LPIP:
- TTX means TTX + Standard of Care. Placebo means placebo + Standard of Care.
- BPI means Brief Pain Inventory which is a medical questionnaire used by patients to measure the severity of their pain.
Phase III CRP: TTX reduced opioid consumption
- There were more patients that reduced their opioid consumption of at least 50% in the TTX treatment group over the Placebo group in both Early Post Injection Period (“EPIP”) and Late Post Injection Period (“LPIP”)
- Furthermore, using any percentage in reduction of opioid consumption, there were more patients in the TTX group who reduced their opioid intake during the clinical trial than in the Placebo group
Early Post Injection Period (Days 5-8)
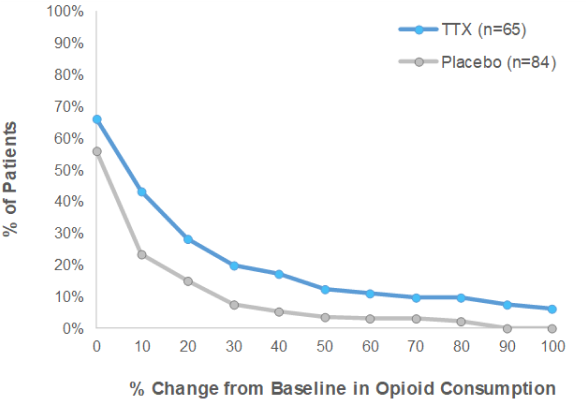
Late Post Injection Period (Days 9-15)
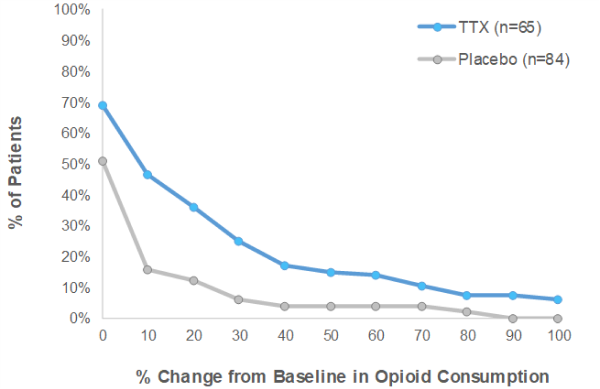
TTX means TTX + Standard of Care. Placebo means placebo + Standard of Care. Standard of Care is defined as optimized opioid and co-analgesic therapy specific to each patient.
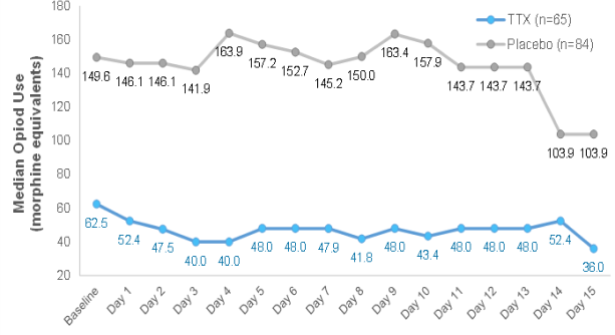
Phase III CRP: TTX Reduced Daily Opioid Use1
- Patients in the TTX group demonstrated an immediate and consistent reduction in opioid use
- Highly encouraging evidence that patients on TTX experienced sufficient pain reduction to reduce their daily use of opioid pain medication
Median patient opioid use by day2
(in Morphine equivalents)
% change of opioid use from Baseline2
by day
(in Morphine equivalents)
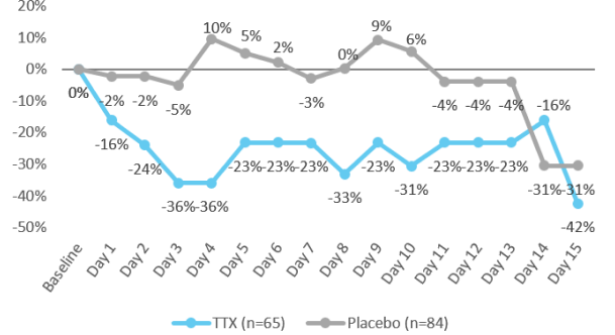
- Placebo group: no meaningful reduction in daily opioid consumption for most of study period
- TTX patients: demonstrated meaningful and sustained reduction in daily opioid consumption
- Opioid use is defined as the average dose, converted to morphine equivalents, as reported on the patient diary, during each treatment period.
- In the Intent to Treat population
Phase III CRP: Global Impression of Change
- Patients in the TTX group reported an improvement in pain compared to the placebo group
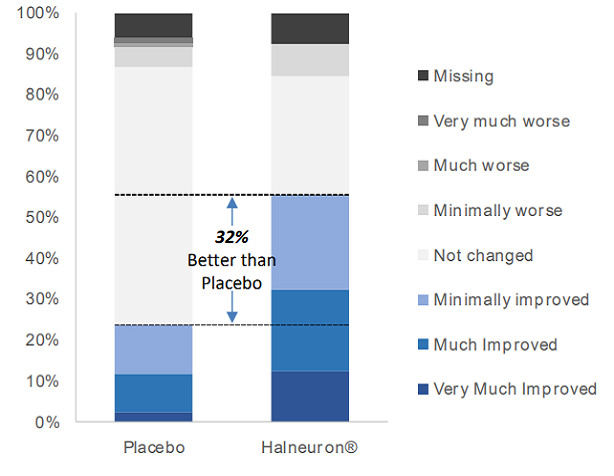
| Halneuron1 | Placebo2 | Difference | |
|---|---|---|---|
| Very Much Improved | 12% | 2% | |
| Much Improved | 20% | 10% | |
| Minimally Improved | 23% | 12% | |
| Total Improved | 55% | 24% | 32% |
| Not changed | 29% | 63% | |
| Minimally worse | 8% | 5% | |
| Much worse | 0% | 1% | |
| Very Much worse | 0% | 1% | |
| Missing | 8% | 6% |
- 55% of patients on TTX reported improvement vs 24% of patients on placebo
- 70% of patients on placebo reported no change or worse pain vs 37% of patients on TTX
- TTX + Standard of Care.
- Placebo + Standard of Care.
- Standard of Care is defined as optimized opioid and co-analgesic therapy specific to each patient.
Phase III CRP: Long duration of pain relief
- After a single cycle of treatment, Responders1 in the TTX group showed a prolonged duration of pain relief that was substantially longer than Responders2 in the Placebo group
- Average number of days of pain response was 57.7 days vs 10.5 days for the TTX vs placebo groups, respectively
- 9 patients (27%) in the TTX group responded for 30 days or longer after the initial injection period
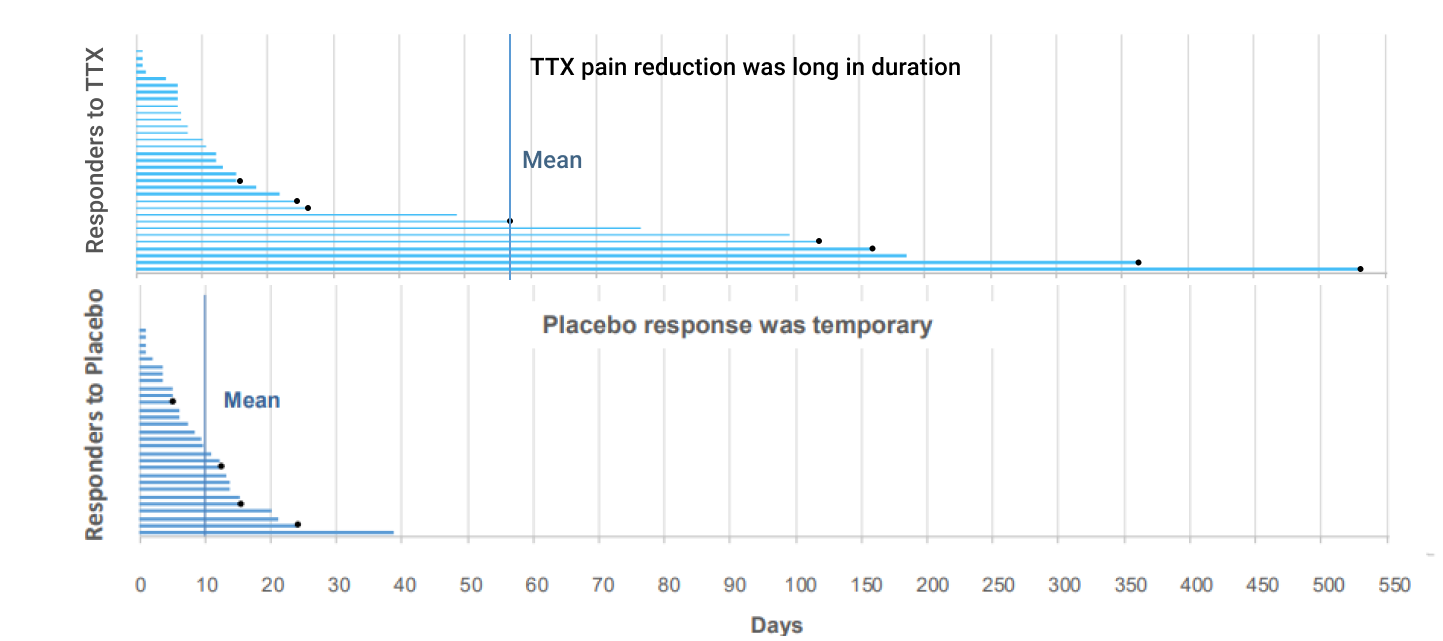
Some patients demonstrated pain reduction for more than 30 days after 1 cycle of treatment.
Most painkillers today require daily intake.
- TTX + Standard of Care
- Placebo + Standard of Care.
- Standard of Care is defined as optimized opioid and co-analgesic therapy specific to each patient.
Phase II CINP: Trial summary
- WEX completed a Phase II CINP study with TTX
- Randomized, double-blind, dose-finding, placebo-controlled, multicenter study of the potential efficacy and safety of TTX in patients with Chemotherapy-Induced Neuropathic Pain (CINP)
- Objective
- Primary objective was to identify up to 2 doses or regimens of TTX for Phase III evaluation
- Secondary objective was to determine the safety and tolerability of multiple doses / regimens of TTX
- Procedures
- Testing period various doses for 4 days and measurement over 4 weeks
- Total of 125 patients in 5 cohorts (4 groups with different TTX dosage levels and 1 placebo group)
- Results
- Dosage of 30 µg twice per day for 4 days demonstrated highest level of pain reduction vs placebo
- This trial demonstrated an acceptable safety profile in patients with CINP.
Responder Analyses: 30% reduction in average NPRS1 score from baseline to any 10 consecutive days
| TTX2 | Placebo3 | |
|---|---|---|
| Yes | 15 (58%) | 8 (32%) |
| No | 11 (42%) | 17 (68%) |
| P-Value | 0.027 | |
| Odds Ratio (vs Placebo) | 3.9 | |
| 95% Cl for odds Ratio | (1.08,14.09) |
- Numeric Pain Rating Scale.
- TTX + Standard of Care.
- Placebo + Standard of Care.
- Standard of Care is defined as optimized opioid and co-analgesic therapy specific to each patient.
Patient Safety
Side effects are mild to moderate and are confined to the injection period.
- Sample includes all patients from: (1) Phase II CINP, (2) Phase II CRP, and (3) Phase III CRP trials.
- Clinical trials undertaken to date have not reported any TTX related severe or life-threatening side effects.

Results from Phase II and III trials demonstrated that Halneuron® has an acceptable safety profile.
Summary of side effects / adverse events (AE) in clinical trials
| Most Common (≥10%) AEs | TTX | Placebo | Difference |
|---|---|---|---|
| Sample Size | 224 | 166 | 58 |
| Gastrointestinal Conditions | |||
| Hypoaesthesia oral | 45% | 9% | 36% |
| Nausea | 38% | 19% | 19% |
| Paraesthesia oral | 30% | 30% | 0% |
| Vomiting | 16% | 8% | 8% |
| Nervous System Disorders | |||
| Dizziness | 34% | 17% | 17% |
| Hypoaesthesia | 25% | 14% | 11% |
| Paraesthesia | 26% | 11% | 15% |
| Somnolence | 10% | 11% | -1% |
| Headache | 17% | 20% | -3% |
| General Disorders & Administration Site Conditions | |||
| Injection site irritation | 18% | 29% | -11% |
| Fatigue | 17% | 10% | 7% |
| Injection site pain | 12% | 20% | -8% |
| Injection site burning | 2% | 9% | -7% |