A compound is labeled a toxin if it elicits an adverse biological reaction when introduced to another organism. However, this is a limited understanding of how beneficial toxins can be. In this series, we aim to demystify toxins, and show how many can be essentially harmless, and even beneficial.
The need for safe and effective pain medication has never been more urgent. As the opioid crisis ravages communities – creating cycles of addiction, suffering, and death – the development of non-opioid analgesics (pain medications) is crucial to ensure those living with pain get the help they need, without the side effects associated with traditional pain treatments.
In our previous articles, we have explored the commonality, safety, and utility of certain toxins. Here, we discuss Tetrodotoxin, a toxin known for its analgesic properties, and the active ingredient in WEX Pharmaceuticals product Halneuron®. While the negative connotations surrounding a medical product containing a “toxic” compound will not disappear overnight, we will be discussing its efficacy and safety when compared to opioids and other toxins.
TTX: A Breakthrough in Non-Opioid Analgesics
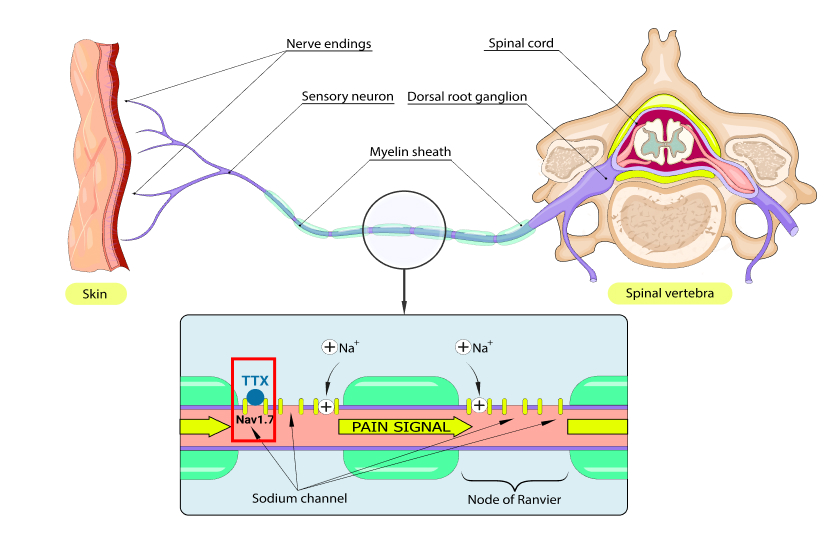
Tetrodotoxin (TTX) is referred to as a neurotoxin – a molecule that has the potential to affect the nervous system at certain doses – found in certain marine and terrestrial life.[i] Accidental ingestion of TTX can be dangerous, but in optimized doses administered during clinical studies, it has been shown to have well-documented analgesic properties with a promising safety profile.
In scientific terms, TTX is what is known as a “sodium channel blocker.” Sodium channels are protein structures found on the surface of nerve cells that regulate the flow of sodium ions into the nerve. These channels allow sodium ions to flow into the nerve cell when they are activated, creating nerve impulses. There are many types of sodium channels in the body, but the sodium channel Nav 1.7 is involved in the transmission of pain signals. As a sodium channel blocker, TTX reduces the flow of sodium ions in these channels, reducing these signals.
TTX’s Clinical Progress
Halneuron® is still in the testing process, but the results found in these studies – including its efficacy in treating cancer pain, burn pain, and chemotherapy-induced neuropathic pain (CINP) – have shown a tremendous ability to mitigate different types of pain in clinical settings. Some key findings from these studies include:
Promising reduction in pain
In a trial studying TTX’s efficacy in treating CINP, patients experienced a 30% or greater reduction in pain,[ii] with TTX when other analgesics and opioids were shown to be ineffective. This highlights how TTX may one day be a viable alternative to opioid pain management of serious, debilitating conditions.
Low incidence of side effects
TTX, when administered in therapeutic doses, exhibited an encouraging safety record. While respiratory depression, euphoria, and gastrointestinal issues are all major side effects of opioid medications, the most prominent side effects found in TTX trials have so far been brief, mild-to-moderate tingling and numbness in the mouth, fingers, and toes, a comparably benign experience for patients.[iii]
Lack of tolerance development
Patients often build tolerance to opioid medications, necessitating higher and riskier doses for the same level of pain relief. In addition, for some, the euphoria associated with opioid use increases the risk that such medications will be used recreationally. TTX’s mechanism of action has demonstrated no tolerance development, making it non-habit forming, easier to stop, and allows patients to remain at the same dosing level throughout treatment.[iv]
TTX: A Big Step To Demystifying Toxins
This series has aimed to shed toxins from their wholly negative labels. While it is important to be aware and cautious of toxins in the abstract, the truth is that there are many toxins that not only are functionally harmless to humans, but also beneficial in a myriad of ways. Tetrodotoxin is just one example of a toxin that can be used for medical applications, but its success in clinical studies highlights its unique potential in demystifying toxins altogether. With each successive study, more patients, researchers, and members of the public will be able to see TTX’s potential as a safe, effective opioid alternative, and a clear example for why the word ‘toxin’ should not be inherently feared.
[i] Narahashi, Toshio. “Tetrodotoxin: a brief history.” Proceedings of the Japan Academy. Series B, Physical and biological sciences vol. 84,5 (2008): 147-54. doi:10.2183/pjab.84.147
[ii] Goldlust, Samuel A., et al. “Tetrodotoxin for Chemotherapy-Induced Neuropathic Pain: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Dose Finding Trial.” Toxins, vol. 13, no. 4, 2021, p. 235, https://doi.org/10.3390/toxins13040235.
[iii] Goldlust, Samuel A., et al. “Tetrodotoxin for Chemotherapy-Induced Neuropathic Pain: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Dose Finding Trial.” Toxins, vol. 13, no. 4, 2021, p. 235, https://doi.org/10.3390/toxins13040235.
[iv] Goldlust, Samuel A., et al. “Tetrodotoxin for Chemotherapy-Induced Neuropathic Pain: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Dose Finding Trial.” Toxins, vol. 13, no. 4, 2021, p. 235, https://doi.org/10.3390/toxins13040235.
DISCLAIMER:
Halneuron® is still being developed and tested in clinical trials and has not yet been approved for sale by Health Canada, the United States Food & Drug Administration (US FDA), or any equivalent medical authority. This article contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995 and forward-looking information under applicable Canadian securities laws (collectively “forward-looking statements”), including statements regarding the safety and therapeutic utility of Halneuron® and Tetrodotoxin (TTX) as a peripheral-acting, non-opioid analgesic. Statements in this document regarding future expectations, beliefs, goals, plans, or prospects constitute forward-looking statements that involve risks and uncertainties, which may cause actual results to differ materially from the statements made. For this purpose, any statements that are contained herein that are not statements of historical fact may be deemed to be forward-looking statements. Without limiting the foregoing, the words “believes”, “anticipates”, “plans”, “intends”, “will”, “should”, “expects”, “projects”, “reduces,” “affirms”, “acceptable”, “accepts”, “establishes”, “continued advancement”, and similar expressions are intended to identify forward-looking statements. You are cautioned that such statements are subject to a multitude of risks and uncertainties that could cause actual results, future circumstances, or events to differ materially from those projected in the forward-looking statements. These risks include, but are not limited to: those associated with the success of research and development programs, the Company’s ability to raise additional funding and the potential dilutive effects thereof, the regulatory approval process, competition, securing and maintaining corporate alliances, market acceptance of the Company’s products, the availability of government and insurance reimbursements for the Company’s products, the strength of intellectual property, reliance on subcontractors and key personnel and other risks detailed from time-to-time in the Company’s public disclosure documents and other filings with the U.S. Securities and Exchange Commission and Canadian securities regulatory authorities. Forward-looking statements are developed based on assumptions about such risks, uncertainties and other factors, including, but not limited to: obtaining positive results of clinical trials, obtaining regulatory approvals, TTX is a more potent analgesic than standard analgesics, safety of product, effectiveness of drug, general business and economic conditions, the Company’s ability to successfully develop and commercialize new products, the assumption that the Company’s current good relationships with third parties will be maintained, the availability of financing on reasonable terms, the Company’s ability to attract and retain skilled staff, market competition, the products and technology offered by the Company’s competitors, no known competing drugs specifically for CINP, and the Company’s ability to protect patents and proprietary rights. Forward-looking statements are made as of the date hereof, and the Company disclaims any intention and has no obligation or responsibility, except as required by law, to update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
